Advancing together in the journey for clinical excellence.

ECN stands as your premier partner in clinical research, offering a network of specialized research sites tailored for efficiency and excellence.
Our method integrates leading-edge strategies in contract, budget, recruitment, and quality control to ensure your clinical trials not only start swiftly but also adhere to the highest standards.
By choosing ECN, you’re choosing a partner committed to the success and quality of your clinical research.
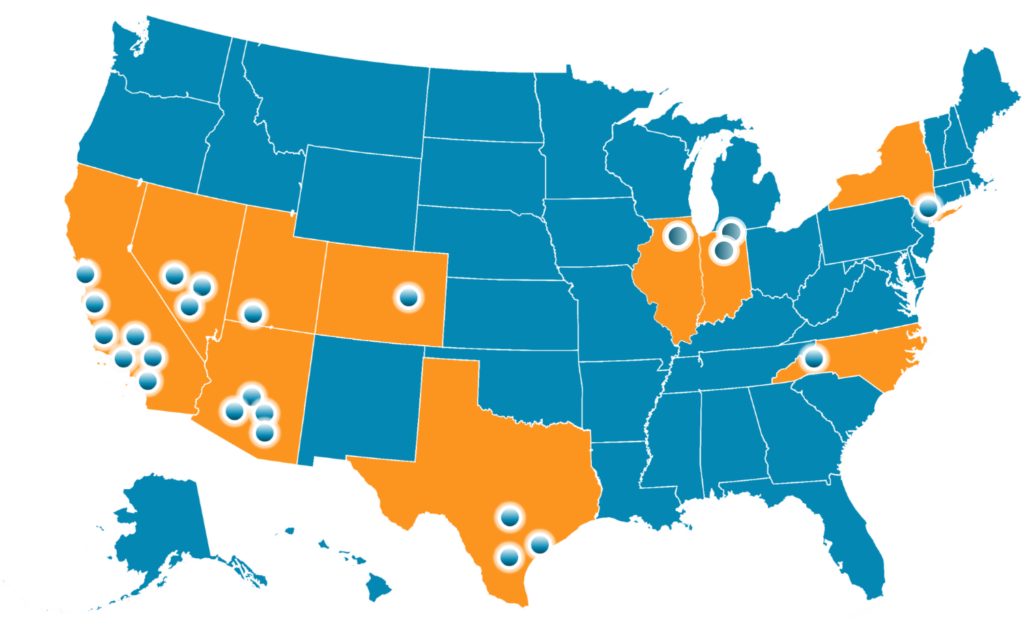
Primarily located in the South/Southwest, ECN is rapidly expanding across the US, ensuring broad demographic coverage for studies.
Our sites are strategically located on hospital campuses or nearby, chosen for their accessibility and proximity to diverse communities.
ECN partners with multi-specialty groups across the US, including surgical centers and hospitals, and had a 15-bed Phase 1 facility for FIH research.

Straightforward contracting, including SOPs.
Quick equipment delivery and site preparation, followed by thorough staff training.
Study Initiation
Efficient coordination of sponsor site visits and quick approvals.

Careful planning of assessments, protocol workflow, and adverse event reporting.

Active ECN recruitment complemented by provider referrals.

Regular internal audits and continuous staff training.
A commitment to enhancing processes through the ECN escalation procedure.

From inception to completion, experience seamless operations.

Expedite study start-up without sacrificing quality.

Leverage efficient practices for optimal financial outcomes.

Accelerate patient enrollment, reducing timelines.

Let us handle the complexities, freeing you to concentrate on the science.

Elevate patient experiences through our refined processes.
Unlock your research’s potential with ECN’s partnership. Dive into a world of expertise, innovation, and full-scale support for your clinical trials.
Together, let’s achieve remarkable outcomes and elevate patient care.